CiPA Initiative
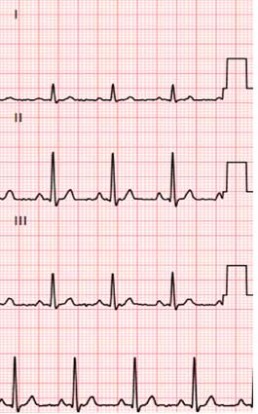
The objective of the CiPA initiative is to facilitate the adoption of a new paradigm to assess the clinical potential of Torsades de pointe, which cannot be measured exclusively by potency of hERG block and not at all by QT prolongation.
The Comprehensive In Vitro Proarrhythmia Assay is currently being designed by expert working groups from:
FDA
Food and Drug Administration
HESI
Health and Environmental Sciences Institute
CSRC
Cardiac Safety Research Institute
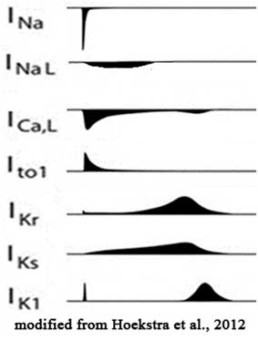
- Develop voltage clamp protocols for core set of cardiac ion channel types responsible for both the depolarization and the repolarization process of cardiac action potential.
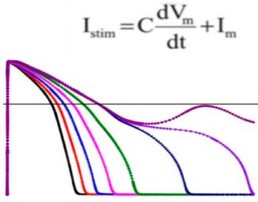
- Develop consensus in silico model to reconstruct electrophysiologic activity within a heart cell
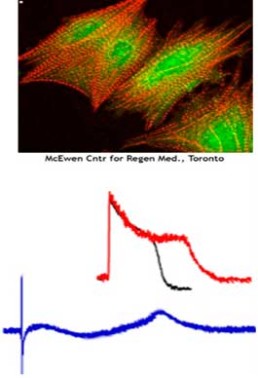
- Investigate capabilities of human stem-cell derived cardiomyocyte assays to confirm findings of in vitro and in silico assays.