Human iPSC Cardiomyocytes

Human cardiomyocytes derived from iPS cells for preclinical drug studies
Human cardiomyocytes derived from induced pluripotent stem cells (hiPSC-CM) provide rapid, reproducible and sensitive predictions of QT prolongation as recommended by the CiPA initiative. Besides QT prolongation, it is also an integrative system that could easily identify potential cardiotoxic effects of compounds (pro-arrhythmogenic, cytotoxic and contractility worsening). hiPSC-CM have contractile ability with ion channel activity and have been recognized for there potential in human heart disease modeling, therefore preclinical cardiotoxicity evaluation and drug discovery are relevant to anticipate drug-induced cardiac side effects.
Human iPSC Cardiomyocytes & Preclinical Safety Studies
Human cardiomyocytes derived from iPS cells provide the best solution to study the repercussion on ion channels and contractility during preclinical studies. This human in vitro model can be used to evaluate the cardiac safety of newly developed drugs and is currently validated by CIPA initiative for cardiac preclinical safety assessment. As a result, a large number of drugs have been withdrawn during late stage of clinical trials or from the market.
What is the added value of such a study?
- Is a procedure recommended by the CiPA initiative
- Threshold concentration of cardiotoxicity
- To rule out potentials false-positive hERG Blocker and other channels blockers
- Leading to the determination of the safety range versus efficacious concentration
- To anticipate drugs effects on excitation and contraction coupling in human heart
- You obtain your safety margin
Technique
-
- xCELLigence RTCA Cardio ECR platform (48 wells plate format)
- iCell®Cardiomyocytes² (Fujifilm)
- Simultaneous recording of Electric Field Potential (EFP) and Impedance signals on the entire plate:-EFP= Reflecting electrical activity
-Impedance= Reflecting cell contraction
- Short and Long term exposure (up to 72h)
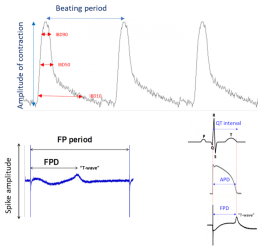
Measured Parameters
- Cell viability: Cell Index
- Electrophysiology (EFP):
Firing rate
FPD as predictor of QT duration
Spike amplitude - Contraction (Impedance): Amplitude of contraction, Beating rate, Individual beat duration (IBD10, 50, 90)
- Proarrhythmic events (BRI)
Main advantages
- Human tissue
- Adjustable to your needs, manage the concentration and replication of each compound in the 48 wells.
- Technically robust
- Highly informative
- Strongly replicable
Reference compounds
- Nifedipine
- E-4031
- Pentamidine
- Dofetilide
- Flecainide
- Mexiletine
- Pilsicainide
Nifedipine (Low TdP risk)
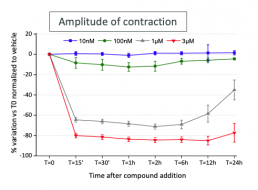
Typical effects of Nifedipine on the amplitude of contraction.
Pentamidine (long-term exposure)
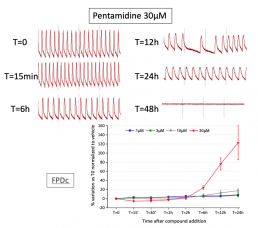
Typical effects of Pentamidine on impedance signal and FPDc.
Results
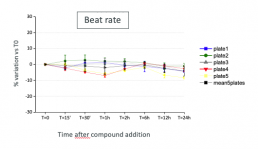
Typical effects of DMSO 0.1% on Beat rate. Stability of recorded parameters in control conditions and reproductibility between plates