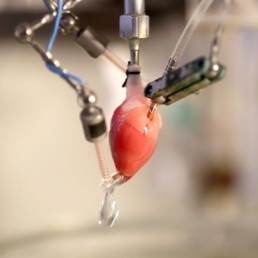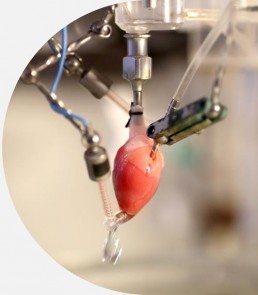
Isolated perfused heart
Langendorff heart studies
The Langendorff isolated perfused heart assay provides rapid, reproducible and sensitive predictions of QT prolongation as recommended The ICH S7B guideline. Besides QT prolongation, Isolated Perfused Heart is also an integrative and native expressed system that could easily identify potential cardiotoxic effects of compounds (pro-arrhythmogenic, cytotoxic and contractility worsening). Therefore, this models could screen selective channel blocker but also multi-channel blocker or indirect channel regulator.
Moreover, this study allows the assessment of the effects of a drug on left ventricular cardiac function (systolic and diastolic function, coronary flow) and cardiac electrical activity (QT prolongation and also all ECG parameters).
Isolated Perfused Heart Assays & Preclinical Safety Studies
Isolated Perfused Heart assays aim to identify potential cardiotoxic effects early in drug discovery phase. ICH Guidelines recommend Isolated Perfused Heart, due to numerous clinically approved drugs reported to increase the pro arrhythmogenic risk (including sudden death) through QT prolongation and left ventricular dysfunction. As a result, a large number of drugs have been withdrawn during late stage of clinical trials or from the market.
What is the added value of such a study?
- Is a procedure recommended by ICH S7B guideline (but not mandatory)
- Threshold concentration of cardiotoxicity
- To rule out potentials false-positive hERG Blocker
- Leading to the determination of the safety range versus efficacious concentration
- You obtain your safety margin
Technique
- Retrograde heart perfusion (Langendorff) at constant pressure with monitoring of haemodynamics and ECGs parameters
- Guinea-pig or rat hearts
- Electrocardiograms, left ventricular pressure parameters and coronary flow are recorded in hearts perfused at a constant pressure (55 ± 5 mmHg)
- At least 3 preparations tested and 3 control preparations (as basic design protocol but can be adapted)
- At least 3 cumulative increasing concentrations of assay compound (at least 4 increasing concentration in order to obtain IC50 value
- Washout period or positive control at the end of the experiment
Measured parameters
- Coronary flow (mL/min)
- Left ventricular pressure parameters: -Developed pressure : maximal systolic pressure, minimal diastolic pressure (mmHg) -Max dP/dt: maximal contraction velocity (mmHg/sec) -Min dP/dt: maximal relaxation velocity (mmHg/sec) -Heart rate: calculated from ventricular contractions (beats/min)
- ECG parameters: -PR, QRS, RR and QT interval (ms) -QTc intervals: Fridericia (QT / (RR)1/3)
- Clinical rhythmology interpretation of the ECG signal, describing events: AV-block, torsade de pointe, ventricular fibrillation, tachycardia…
Main Advantages
- Technically robust
- Cost effective
- Highly informative (more accurate IC50 value)
- Strongly replicable
- Design protocol could be adapted
- Could be performed under GLP conditions
Results
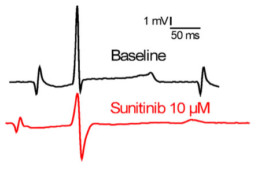
Typical effects of sunitinib on ECGs parameters.
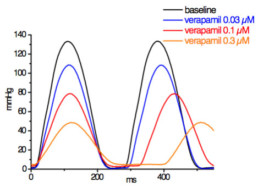
Typical concentration dependent effects of verapamil on LVP parameters.
Reference compounds
- Quinidine
- Dofetilide
- Verapamil
- Sunitinib