Other Potassium channel assays (hKVLQT1/MinK, hKV4.3, hKV1.5) for preclinical safety studies
The three main ion channels of the heart, i.e. hERG, Cav1.2 and Nav1.5 do not completely cover the entire spectrum of electrical heart activity. An inhibition of the other remaining cardiac ion channels could also alter cardiac activity and modify the QT interval. PhysioStim provides a complete range of additional ion channel assays in order to quickly and accurately detect any potential undesirable cardiovascular effects.
hKVLQT1/MinK ASSAY
The hKVLQT1/MinK channel plays an important role in cardiac action potential repolarisation. A pharmacological reduction of the IKs current may delay the repolarisation process and lead to a prolongation of the QT interval. The hKVLQT1/MinK potassium channel is therefore considered as a specific target for cardiac safety assessment.
Technique
- Conventional manual Patch Clamp
- 3 or 4 increasing concentrations of assay compound tested independently
Study model
- Human Embryonic Kidney HEK-293 cells
- 3 to 6 treated cells per concentration tested and 3 to 6 control cells (as basic design protocol but can be adapted)
Measured parameters
- Amplitude of the peak current upon repolarization to –40mV (pA)
- Amplitude of the base current at –80mV (pA)
- Inhibition of hKvLQT1/MinK peak current amplitude (%)
- IC50 value
Stimulation protocol
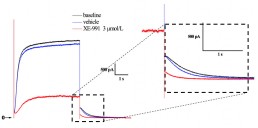
Typical effects of XE-991 on IKs currents recorded from HEK-293 cells.
Reference compounds
Reference CompoundsIC₅₀
XE-9911.5 µM
Chromanol 293B6.0 µM
Mefloquine0.7 µM
hKv4.3 ASSAY
Since the Kv4.3 potassium channel plays an important role in cardiac action potential repolarisation, a pharmacological reduction of the Ito current may delay the repolarisation process and may lead to a prolongation of the QT interval. Thus the Kv4.3 potassium channel represent a specific target for the cardiac safety assessment.
Technique
- Conventional manual Patch Clamp
- 3 or 4 increasing concentrations of assay compound tested independently
Study model
- Chinese Hamster Ovary CHO cells
- 3 to 6 treated cells per concentration tested and 3 to 6 control cells (as basic design protocol but can be adapted)
Measured parameters
- Amplitude of the peak current upon depolarization to +40 mV (pA)
- Amplitude of the base current at –80 mV (pA)
- Inhibition of Kv4.3 peak current amplitude (%)
- IC50 value
Stimulation protocol
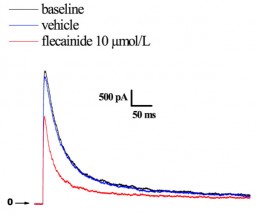
Typical effects of flecainide effects on Ito currents recorded from CHO cells.
Reference compounds
Reference CompoundsIC₅₀
Flecainide19.6 µM
hKv1.5 ASSAY
The human Kv1.5 potassium channel encodes for the ultrarapid component of the delayed rectifier potassium channel responsible of IKur current. In the cardiac tissue, Kv1.5 potassium channels are only expressed in atria in which they represent a major component of the cardiac repolarisation of atrial action potential. Thus Kv1.5 channel represents a major target for the treatment of atrial fibrillation, the most common sustained arrhythmia.
Technique
- Conventional manual Patch Clamp
- 3 or 4 increasing concentrations of assay compound tested independently
Study model
- Human Embryonic Kidney HEK-293 cells
- 3 to 6 treated cells per concentration tested and 3 to 6 control cells (as basic design protocol but can be adapted)
Measured parameters
- Amplitude of the peak current upon depolarization to +50mV (pA)
- Amplitude of the base current at –80 mV (pA)
- Inhibition of hKv1.5 peak current amplitude (%)
- IC50 value
Stimulation protocol
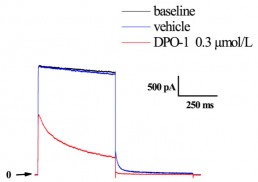
Typical effects of DPO-1 on IKur currents recorded from HEK-293 cells
Reference compounds
Reference CompoundsIC₅₀
PSORA-45.5 nM
DPO-168.0 nM
KN-930.6 µM