Cardiac toxicity comparison of Roundup® and glyphosate
Cardiac toxicity comparison of Roundup® and glyphosate on human Induced Pluripotent Stem cells derived cardiomyocytes
Printemps R, GuilbotS, Le Grand M, PhysioStim, Zone Industriellede Brénas, 81440 Lautrec, France.
Although pesticides are known to be key actors of modern agriculture,they are source of controversies due to their suspected hazardous health effects.
Roundup®, one of the most popularized pesticide, is an herbicide formulation based on an active ingredient, glyphosate, and adjuvants. The broad use of Roundup® through they ears in agricultural practices as well as home garden care led to large exposure of humans and mammalians to glyphosate. Evaluation of the safety and potential health risks from exposure to pesticides remains a major challenge.
Roundup® and glyphosate were compared using human cell based model to evaluate their impacts on cardiovascular function after exposition.
Materials and Methods
- iCell® Cardiomyocytes2 were supplied by Cellular Dynamics (a Fujifilm company) and seeded on 48 well plate following manufacturers instruction and analyzed using the xCELLigence RTCA cardio ECR platform (simultaneous measurement of MEA and impedance).
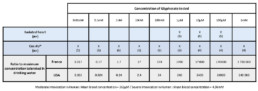
- 4 days after seeding, iCell2 cardiomyocytes were exposed during 24h to 5 different concentrations of Roundup® or glyphosate(from 0.01 to 100μM) prepared from stock solutions diluted in distilled water(final concentration of distilled water was 0.3%).
- Roundup® solution used during this study is composed of 400g/L of glyphosate. Concentration of Roundup® presented here after correspond to the concentration of glyphosate present in the solution (Roundup® 10μM correspond to 10μM of glyphosate)
- Data are expressed as Mean ± SD. Each concentration of compound was administrated in 4 wells.
- The following parameters were monitored: Cell Index (CI), Amplitude of contraction, Beat rate (BR),Beating period(BP),Individual Beating Duration(IBD),Field Potential Duration(FPD) and FPD corrected by Fridericia(FPDc),Spike amplitude,Beating Rhythm Irregularity (BRI).
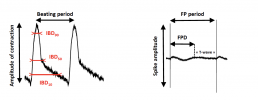
Results
Roundup®

Glyphosate


- No effect of glyphosate from 0.01 μM to 100 μM on viability, contractility and electrical activity.
- Roundup® up to 10 μM had no effect on viability and electrical activity, only as light decrease of amplitude of contraction was recorded (-11%) after 6 hand sustained within 24h at 10μM.
- iPSC-CMs stopped beating during Roundup® 100μM.
conclusion
- The absence of effects of glyphosate on cardiac function indicates that the cardiotoxicity observed with Roundup®could be attributed to Roundup®’sadjuvants.
- This predictive and sensitive assay can be very useful for cardiotoxicity assessment of pesticides using a human cell based model. This work demonstrates that the evaluation of pesticides’ adjuvants is necessary to better address the safety of pesticides which can also be enlarged to a broader class of molecules.
Please fill out the form below to receive the poster
Human Cardiomyocytes Roundup
Assessment of Roundup® cardiotoxicity on guinea-pig isolated Langendorff perfused heart and human lnduced Pluripotent Stem Cells derived cardiomyocytes
R. Printemps, S. Guilbot, H. Didier, M. Le Grand, PhysioStim, Zone Industrielle de Brénas, 81440 Lautrec, France
Although pesticides are known to be the key actors of modern agriculture, they are source of controversies due to their suspected hazardous health effects. Roundup®, one of the most popularized pesticide, is composed of Glyphosate, its active ingredient, and adjuvants. The broad use of Roundup® through the years in agricultural practices as well as home garden care led to large exposure of humans and mammalians to Glyphosate.
Many pesticides such as Glyphosate persist in soils and water so that human exposure can be important even to those who do not use it. ln France, the Maximum Contamination Level (MCL) authorized in drinking water is 0.1 ng/ml (=0.59nM) while in USA, EPA authorized concentration up to 0.7ng/ml (=4.lnM). Acute intoxication after ingesting Glyphosate are also observed in suicidai or accidentai cases leading to mean blood concentration ranging from 61µg/ml (=361µM) considered as moderate intoxication to 838µg/ml (=4.96mM) considered as severe intoxication.A number of severe clinical outcomes have been reported: nausea, vomiting, ulceration, acidosis, respiratory distress, impaired renal function, hepatic toxicity, haemodynamic disturbance, cardiac arrhythmia. The purpose of this work was to examine the effects of Roundup® exposition on heart function using Langendorff isolated guinea-pig (GP) perfused heart and human lnduced Pluripotent Stem Cells derived cardiomyocytes (hiPSC-CM) models.
Methods
lsolated Langendorff-perfused guinea pig heart preparations
- Spontaneous beating Langendorff isolated perfused hearts from male guinea-pig were perfused with Krebs' solution, oxygenated and warmed at 37 + 1 °C, at a constant perfusion pressure of 55 + 5 mm Hg.
- After an equilibration period and a 10 min contrai perfusion, three concentrations of Glyphosate (see table 1) were perfused during 20 min each.
- The following parameters were measured: left ventricular pressure (LVP), maximal contraction velocity (Max dP/dt) and maximal relaxation velocity (Min dP/dt), heart rate (HR), coronary flow (CF) and ECG parameters (PR, RR, QRS, QT and QTc (Fridericia) intervals)
Hum an lnduced Pluripotent Stem cells derived cardiomyocytes
- Cor.4U® Cardiomyocytes were supplied by Ncardia and seeded on 48 wells plate following manufacturers instruction and analyzed using the xCELLigence RTCA cardia ECR platform (simultaneous measurement of MEA and impedance).
- 4 to 5 days after seeding, Cor.4U® cardiomyocytes were exposed during 18h to 24h to different concentrations of Roundup (up to 9 concentrations, see table 1, only some of them are presented in the result section).
- The following parameters were monitored: Cell Index (Cl), Amplitude of contraction, Beat rate, Beating period, lndividual Beating Duration (IBD), Field Potential Duration (FPD) and FPD corrected by Fridericia (FPDc), Spike amplitude, Beating Rhythm lrregularity (BRI).
Compound administration
Roundup® was diluted in distilled water and the combine effects of Glyphosate and adjuvants were evaluated. Concentration of Roundup® tested have been expressed as Glyphosate concentration.
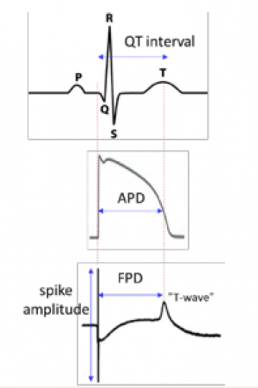
Results
Guinea pig heart preparation


- No effect of Glyphosate at 1µM
- No effect of Glyphosate at 10µM on ECG parameters but , ↗ CF (+39%) and ↘ LVP (-26%)
- At lOOµM, Glyphosate ↘ HR (-33%), LVP (-92%) and CF (-43%) and , ↗ all ECG parameters (RR +50%, PR +39%, QRS +58% and QTc +28%). AV Black was observed in 1 of 5 preparations.
Cor.4U ® cardiomyocytes


- No effect of Glyphosate from 0.01n M to lµM on viability, contractility and electrical activity.
- At lOµM, Glyphosate had no effect on Cell index and contractility but ,↗ FPDc (+15%).
- Glyphosate induced ↘ Cell Index with time at 100µM (-35% after 6h), ↘ amplitude of contraction and beat rate (maximum effect -48% and -45% after 6h respectively) but no modification of FPDc before 18h exposition.
- With Glyphosate lmM, Cor.4U® cardiomyocytes stopped beating after 5min exposition and Cell Index ↘ up to -97% after 6h corresponding to cardiomyocytes death.
conclusion
- Evaluation of the global effects of Roundup® (Glyphosate + adjuvants).
- Bath models were able to detect cardiotoxicity of Roundup® mainly characterized by a massive decrease of the heart's contractile function and troubles in electrical conduction.
- Effects observed in man after Roundup® intoxication, as reported in the literature (moderate ≈ 360µM to severe intoxication ≈ 5mM), were consistent with those observed in these in vitro - ex vivo models.
- These predictive and sensitive assays are particularly relevant for cardiotoxicity assessment of pesticides but also to many other compounds produced by chemicals and fine chemicals industries.
Please fill out the form below to receive the poster
Human Cardiomyocytes aconitine resveratrol
Effects of resveratrol on aconitine-induced cardiac arrhythmia in human induced pluripotent stem cell derived cardiomyocytes
R. Printemps, S. Guilbot, M. Le Grand, PhysioStim, Zone Industrielle de Brénas, 81440 Lautrec, France
Resveratrol, a natural ingredient of grape skin, has been demonstrated to exert protective effect on the cardiovascular system. The use of aconitine, the main effective ingredient of a well-known medical herb widely used in traditional Chinese medicine (aconitium), is associated with severe cardiovascular toxicities including tachyarrhythmia and hypotension. The aim of the present work was to evaluate the potential cardioprotective action of resveratrol on aconitine-induced arrhythmia in human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs). To this end, aconitine and resveratrol were tested alone first before evaluation of the co-administration of both compounds to finally assess the impact of a post-treatment of resveratrol after aconitine administration or a pre-treatment of resveratrol before aconitine-induced cardiac arrhythmia.
Methods
Human Induced Pluripotent Stem cells derived cardiomyocytes (iPSC-CMs)
- iCell® Cardiomyocytes2 were supplied by Cellular Dynamics (a Fujifilm company) and seeded on 48 wells plate following manufacturers instruction and analyzed using the xCELLigence RTCA cardio ECR platform (simultaneous measurement of MEA and impedance).
- 4 to 6 days after seeding, iCell2 cardiomyocytes were exposed to different concentrations of aconitine, resveratrol as well as the combination of both compounds, whether by co-administration or by pre-treatment or post-treatment with resveratrol.
- Data are expressed as Mean ± SD and the first 2h of treatment are presented. This study is composed of 5 groups and the number of replicates in each group is detailed in the experimental design below.
- The following parameters were monitored: Cell Index (CI), Amplitude of contraction, Beat rate (BR) , Beating period (BP) , Individual Beating Duration (IBD), Field Potential Duration (FPD) and FPD corrected by Fridericia (FPDc), Spike amplitude, Beating Rhythm Irregularity (BRI).
Experimental design
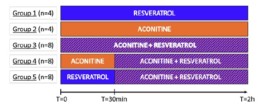
Results
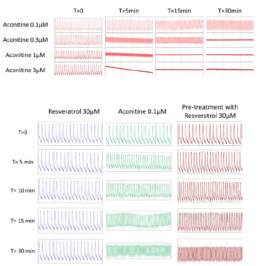
- After 30min, the cells stopped beating with aconitine 0.3µM, 1µM and 3µM.
- Several concentrations of resveratrol were evaluated (0.1µM, 1µM, 10µM and 30µM), no major modification of all the parameters recorded was observed (data not shown).
- Aconitine 0.1µM will be the concentration selected to induce arrhythmia.
- Resveratrol 30µM will be evaluated for its antiarrhythmic capacity to reverse aconitine’s effects.
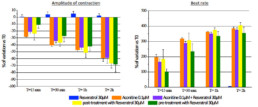
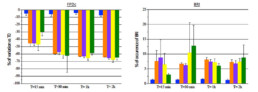
- Tested alone, resveratrol 30µM had no effect on hiPSC-CMs while aconitine 0.1µM time-dependently ↘ amplitude of contraction (-40% after 30min) and FPDc (-60% after 30min) and ↗ Beat rate (+318% after 30min) and BRI (x5 times vs resveratrol).
- Compared to aconitine 0.1µM alone, co-administration of aconitine 0.1µM + resveratrol 30µM had no effect on FPDc and BRI and only slightly lowered effects on amplitude of contraction (∆= -6% after 30min) and Beat rate (∆= -29% after 30min).
- Post-addition of resveratrol 30µM after 30-min exposition to aconitine 0.1µM did not reduce aconitine 0.1µM effects (Amplitude of contraction: ∆= -4%, Beat rate: ∆= -11%, FPDc: ∆= +1% after 30min respectively).
- A 30 min pre-treatment with resveratrol 30µM ↘ aconitine’s effects on all parameters (amplitude of contraction ∆= -17%, beat rate ∆= -93%, FPDc ∆= -15%, BRI ÷3.8 after 15min) and the occurrence of aconitine’s normal effects are delayed by 30min.
conclusion
- Exposition of hiPSC-CMs to aconitine induce a massive increase of beating frequency and a decrease of amplitude of contraction.
- In the same time, resveratrol was not able to suppress aconitine-induced arrhythmia in hiPSC-CMs. Resveratrol was only able to slightly reduce and also delay the occurrence of the maximum effects observed in the presence of aconitine when administrated as a pre-treatment.
- This multi-parametric assay is particularly relevant for the evaluation of drugs’ anti-arrhythmic properties in a model of cardiac arrhythmia in Human iPSC derived cardiomyocytes.
Please fill out the form below to receive the poster
Human Cardiomyocytes Pentamidine Pilsicainide
Evaluation of two hERG channel trafficking modulators, pentamidine and pilsicainide, on stably transfected cell line and human induced pluripotent stem cell-derived cardiomyocytes
R. Printemps1, S. Guilbot1, M. Morton2, M. Le Grand1 – 1 PhysioStim, Zone Industrielle de Brénas, 81440 Lautrec, France | 2ApconiX Ltd, 3F68 AlderleyPark, Cheshire, SK104TG, United Kingdom
hERG trafficking inhibition is known to be one of the mechanisms of indirect hERG inhibition, resulting in QT prolongation and lethal arrhythmia. An increasing number of drugs have been shown to interfere with hERG trafficking. Pilsicainide is a widely used antiarrhythmic agent (class Ic) especially for treating atrial fibrillation. This compound has been evaluated on hERG channel current expressed in stably transfected mammalian cell line as well as on human induced pluripotent stem cell derived cardiomyocytes (iPSC-CM) and compared to pentamidine, a well-known hERG trafficking inhibitor.
Method
Ion channel data
- Electrophysiological recordings of hERG, Nav1.5 (peak), Ito and Iks currents were made from Chinese Hamster Ovary (CHO) cell lines while Cav1.2 currents were made from Human Embryonic Kidney (HEK-293) cell line. All cell lines stably expressed the full-length ion channel. Single cell ionic currents were measured in whole-cell configuration at room temperature (21-23⁰C) using a Patchliner (Nanion Technologies).
- For acute study, cells were clamped at a holding potential of -80mV before a depolarizing step appropriate for each channel. Six Point concentration-response curves were generated by cumulative addition of compound and steady-state inhibition was achieved before the next concentration of compound was added.
- During chronic exposure, hERG cells were exposed during 24h to different concentrations of pentamidine or pilsicainide and then hERG current amplitudes were recorded.
- Data are expressed as Mean ± SEM. For acute study, experiments were replicated 4 times while during chronic exposition, the number of experiments ranged from 28 to 44 for pentamidine, and between 90 and 165 for pilsicainide.
Human Induced Pluripotent Stem cells derived cardiomyocytes (iPSC-CM)
- iCell® Cardiomyocytes2 were supplied by Cellular Dynamics (a Fujifilm company) and seeded on 48 wells plate following manufacturers instruction and analyzed using the xCELLigence RTCA cardio ECR platform (simultaneous measurement of MEA and impedance).
- 4 to 6 days after seeding, iCell2 cardiomyocytes were exposed during 48h to different concentrations of pentamidine and pilsicainide.
- The following parameters were monitored: Cell Index (CI), Amplitude of contraction, Beat rate (BR) , Beating period (BP) , Individual Beating Duration (IBD), Field Potential Duration (FPD) and FPD corrected by Fridericia (FPDc), Spike amplitude, Beating Rhythm Irregularity (BRI).
Results
Ion channel experiments
Ion channel panel, acute exposition:
hERG channel
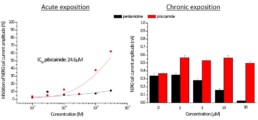
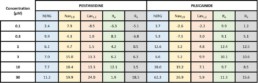
- No acute effect of pentamidine on hERG, Nav1.5, Cav1.2, Iks and Ito.
- Chronic exposition to pentamidine decrease the amplitude of hERG current (estimated IC50 = 9.0µM)
- Acute effect of pilsicainide on hERG channel (estimated IC50 = 24.6µM) but no acute effects on Nav1.5, Cav1.2, Iks and Ito.
- Chronic exposition to pilsicainide increase the amplitude of hERG current (≈+50%) whatever the concentration tested.
Ion channel panel, acute exposition:
iPSCs cardiomyocytes

Pentamidine

Pilsicainide

- No acute effect of pentamidine on each parameter during the first 2 hours. From 6h to 24h, pentamidine 30 μM time-dependently ↓ amplitude of contraction, BR and CIand ↓ FPDc (+123% at 24h) and BRI (at 12h). After 48h, the cells stopped beating with pentamidine 30μM.
- Acute effects of pilsicainide conduct to a concentration-dependent ↓ of BR (-28% at 10µM) and an ↑ of FPDc (+28% at 10µM) with maximum effect after 15 min and sustained through 48h. Pilsicainide from 1 to 10µM had no effect on amplitude of contraction and CI. The cells stopped beating immediately after pilsicainide 20µM addition.
conclusion
- Absence of pentamidine’s acute effects on hERG current in CHO cells is confirmed in Human iPSC-CM. Indeed, no modification of FPDc was observed before 6h of treatment at the highest concentration tested (30 µM).
- On the other hand, the increase of hERG trafficking observed on CHO cells by pilsicainide was not confirmed in iPSC-CM as FPDc was prolonged after short-term exposition and maintained during time.
- This predictive and sensitive assay is particularly relevant for long-term cardiotoxicity assessment in Human iPSC derived cardiomyocytes.
Please fill out the form below to receive the poster
Preclinical tool for CARDIAC SAFETY assessment
Preclinical tool for CARDIAC SAFETY assessment
Stéphanie Guilbot – European xCELLigence User Meeting, November 21, 2019
A preclinical CRO
- Scientific team graduated in cardiac electrophysiology
- Reliable and high level of expertise in cardiac safety
- TGLP compliant since 2001
- Standardized and customized full services to optimize the derisking of compounds
- Preclinical studies according to ICH regulations and health authorities
- Animal welfare : ethic commitee US006/CREFRE
- Qualified for research tax credit
- Our clients: pharmaceutical & biotechnology companies mainly in Europe
Fill out the form below to receive the scientific presentation by email
IKs Current in Repolarization Reserve
Role of Cardiac IKs Current in Repolarization Reserve Process During Late Sodium Current (INaL) Activation
Richard Printemps1, Céline Salvetat1, Jean-François Faivre2, Marie Le Grand1, Patrick Bois 2, Hamid Moha ou Maati1, 3 — 1 PhysioStim, Zone Industrielle de Brénas, 81440 Lautrec, France | 2 CNRS, ERL 7003, Laboratoire STIM, Université de Poitiers, F-86073 Poitiers, France | 3 Institut de Génomique Fonctionnel IGF CNRS INSERM UMR5203, 141 rue de la Cardonille, F-34090 Montpellier France
ABSTRACT
The slow delayed rectifier K+ current (IKs) mediated by KCNQ1/KCNE1 channels contributes to the cardiac action potential in human and other species. Several studies have shown that IKs protects the heart from excessive action potential prolongation induced by the rapid delayed rectifier K+ current (IKr) inhibition. Moreover, several studies have shown that combined pharmacological inhibition of IKs and IKr currents increases cardiac parameters such as instability and dispersion of repolarization, and short term QT interval variability. It is known these effects promote a high risk of occurrence of « torsade de pointes ». However, the consequences of combined IKs current inhibition with an increase of the late sodium current (INaL) on cardiac repolarization has not been studied and reported in the literature.
The aim of this work is to study the effects of pharmacological inhibition of IKs with chromanol 293B, a reference inhibitor of IKs current, during action potential prolongation promoted by INaL pharmacological activation by veratridine. These effects were also evaluated in the presence of doxorubicin, an anti cancer drug increasing cardiac ventricular repolarisation and QT interval duration.
BIO International Convention 2019 in Philadelphia

BIO International Convention 2019 in Philadelphia
Marie Le Grand, PhysioStim’s CEO, will be present at the BIO International Convention in Philadelphia from June 3rd to June 6th, 2019. Please feel free to join us in the French Pavillon (booth ⧣2109) to discuss all your drug development projects and cardiac safety assays with Marie. See you there!
New Partnership for Comprehensive In Vitro Cardiac Safety Assessment
PhysioStim & ACEA Biosciences announce partnership
A hi-tech alliance to measure preclinical cardiac safety in vitro
ACEA Biosciences and PhysioStim will work together to provide comprehensive in vitro cardiac safety and toxicity assessment services for pharmaceutical and biotechnology companies in Europe.
Press release
ACEA Biosciences Inc. (ACEA), now a part of Agilent Technologies, is a pioneer in the development and commercialization of high performance, cutting-edge cell analysis platforms for life science research. Today, ACEA Biosciences and PhysioStim, a leading European CRO with GLP-certified expertise in cardiovascular pharmacology and electrophysiology, are announcing a partnership to provide comprehensive in vitro cardiac safety and toxicity assessment services for pharmaceutical and biotechnology companies in Europe.
Measuring the cardiac liability of pharmaceutical compounds
Cardiac liability of pharmaceutical compounds is a pervasive issue in drug development that needs to be carefully managed and mitigated. PhysioStim will adopt and incorporate ACEA’s xCELLigence RTCA CardioECR system as an important part of its service offerings together with human induced pluripotent stem cells (hi-PSCs), to assess the cardiac liability of pharmaceutical compounds and biologics on cardiomyocyte contractility, integrated ion-channel activity and longer-term viability, and structural toxicity.
PhysioStim, an expert CRO for preclinical cardiac screening
PhysioStim is one of Europe’s most recognized and reliable CROs offering electrophysiological studies, preclinical resources, and dedicated expertise in evaluating compounds under GLP (Good Laboratory Practices), in accordance with the International Conference of Harmonization (ICH) S7A and S7B guidelines for cardiac safety assessment. More recently, PhysioStim has taken steps to be aligned with the upcoming FDA-sponsored Comprehensive In Vitro Pro-Arrhythmia (CiPA) initiative guidelines. As part of this initiative, compounds are ranked for their pro-arrhythmic liability using a suite of approaches including the screening of compounds against critical cardiac ion channels expressed recombinantly in non-cardiac cell lines, using in silico methodology and utilizing human-derived cardiomyocytes, such as hiPSCs.
ACEA, a pioneer in real time cell assessment
ACEA is the developer of xCELLigence RTCA products with more than 3,000 installations spanning academia, industry and government laboratories. The xCELLigence CardioECR system combines high- frequency measurement of cellular impedance with multi-electrode array technology to simultaneously assess cardiomyocyte contractility, viability, and electrophysiology. The system has distinctive features such as enhanced impedance data acquisition rate (everyone millisecond), simultaneous recording of contractility and electrophysiology and electrical pacing feature all in a microtiter plate format which makes it amenable and attractive for the screening of compounds which may have cardiac liability. The CardioECR system was one of the few selected technologies which were utilized in the validation of compounds as part of the CiPA initiative.
A hi-tech partnership for CIPA-compliant cardiac screening
“We are continuing to build our service capabilities with more predictive assays for assessment of cardiac liability, and incorporation ACEA’s CardioECR system is an example of how we are aligning ourselves for the future with the CiPA guidelines,” stated Dr. Marie Le Grand, CEO of PhysioStim. “The fact that the CardioECR system is not only able to assess integrated ion channel activity in hiPSCs, but also contractility and viability is an added advantage which will allow us to screen compounds for cardiac liability with more confidence.”
As part of this partnership, ACEA will direct and refer all service requests in Europe on the CardioECR system to PhysioStim.
“We are happy to work with world-class electrophysiologists and scientists at PhysioStim to better serve our existing customers and those clients that are eager to learn more about the potential cardiac liability of their compounds,” stated Dr. Abassi, VP of Business Development at ACEA.
About PhysioStim
PhysioStim is an independent preclinical CRO with high-level expertise in the field of cardiovascular pharmacology and electrophysiology. The company was founded in 2000 by Dr. Marie Le Grand, who is the current CEO, and is located in Lautrec in Southern France. PhysioStim aims to address the growing need of the pharmaceutical industry and biotech companies to conduct high-quality preclinical assays and studies. Over the years, PhysioStim has grown into a highly-valued team that rises to the challenge of designing and conducting high-quality studies for drug development and derisking of compounds.
About ACEA Biosciences
Founded in 2002, ACEA Biosciences, Inc., now a part of Agilent Technologies, is a pioneer in the development and commercialization of high performance, cutting-edge cell analysis platforms for life science research. Researchers are advancing their studies utilizing over 3,000 placed instruments for broad and diverse applications. The technology has been cited in over 2000 peer-reviewed publications. ACEA’s xCELLigence impedance-based, label-free, real time cell analysis system and NovoCyte flow cytometers are used in pre-clinical drug discovery and development, toxicity, safety pharmacology, and basic academic research.