hERG assays

hERG
hERG assays under GLP conditions is mandatory study according to ICH S7B. It is also strongly recommanded by PhysioStim’s expert and health authorities to perform non-GLP hERG assays (with automated or manual patch-clamp) as an early assessment during drug discovery phase.
hERG assays are essential to study the cardiovascular safety of new compounds and biologics (new chemical entity, antibody, siRNA, etc) . It’s the pore forming subunit of a potassium channel that conducts the delayed rectifier potassium current known as IKr. This potassium current contributes to the repolarization phase of the action potential. When the hERG channel cannot adequately conduct potassium current across the cell membrane, a fatal disorder called type 2 long QT syndrome (LQT2) and/or Torsade de Pointes could occur.
PhysioStim’s electrophysiologists conducts hERG studies in agreement with all ICH guidelines to perform screening and under GLP-compliance for mandatory studies.
hERG Assays & Preclinical Safety Studies
Preclinical hERG assays aim to identify potential blockade effects early in drug discovery phase. ICH Guidelines state that hERG assays are mandatory, due to numerous clinically approved drugs reported to increase the pro arrhythmogenic risk (including sudden death) through hERG channel blockade. As a result, a large number of drugs have been withdrawn during late stage of clinical trials or from the market.
What is the added value of such a study?
- Is a cardiac ion channel recommended by the CiPA
- Threshold concentration of hERG blockade
- Leading to the determination of the safety range versus efficacious concentration
- You obtain your safety margin
Automated Patch Clamp
Technique
- Up to 6 cumulative increasing concentrations of test compound or biologic.
- Experiment conducted at room temperature or at physiological temperature (35°C)
- Human Embryonic Kidney HEK-293 cells
Measured parameters
- Amplitude of the tail current upon repolarization to -40 mV (pA)
- Amplitude of the base current at -80 mV (pA)
- Ion current amplitude measurement
- Inhibition of hERG tail current (%)
- IC50 value (at least 4 concentrations required)
Main advantages
- High throughput screening
- Cost effective
- Fast process to delivery results
- Perfect at earliest stages
- Design protocol could be adapted
Manual Patch Clamp
Technique
- Up to 4 cumulative increasing concentrations of test compound or biologic.
- Experiment conducted at room temperature or at physiological temperature (35°C)
- Human Embryonic Kidney HEK-293 cells
- At least 3 treated cells are recommended
Measured parameters
- Amplitude of the tail current upon repolarization to -40 mV (pA)
- Amplitude of the base current at -80 mV (pA)
- Ion current amplitude measurement
- Inhibition of hERG tail current (%)
- IC50 value (at least 4 concentrations required)
Main advantages
- Technically robust
- Highly informative (more accurate IC50 value)
- Strongly replicable
- Design protocol could be adapted
Manual Patch Clamp - GLP Conditions
Technique
- Human Embryonic Kidney HEK-293 cells
- Experiment conducted at room temperature or at physiological temperature (35°C)
- 4 increasing cumulative concentrations of test compound or biologic.
- At least 5 treated cells is highly recommended for a regulatory application
- Vehicle group is also highly recommended
- Sampling pre- and post- perfusion for analytical quantification
Measured parameters
- Amplitude of the tail current upon repolarization to -40 mV (pA)
- Amplitude of the base current at -80 mV (pA)
- Ion current amplitude measurement
- Inhibition of hERG tail current (%)
- IC50 value
Main advantages
- Technically robust
- Highly informative (more accurate IC50 value)
- Strongly replicable
- Design protocol could be adapted
- Sampling
- Analytical quantification under GLP conditions (also mandatory) could be performed by the Sponsor or with PhysioStim
Stimulation protocol
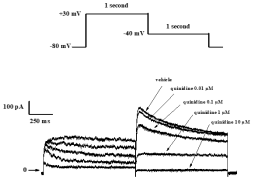
Typical concentration-dependent effects of quinidine on IKr current recorded from HEK-293 cells.
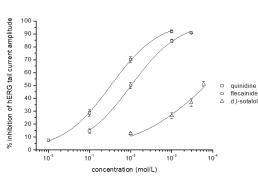
Typical concentration-dependent effects of quinidine, flecainide and d,l-sotalol on IKr current recorded from HEK-293 cells.
Reference compounds or positive controls
Reference Compounds hERGIC₅₀
E–403132.3 nM
Dofetilide10.8 nM
Flecaïnide1.0 µM
Sotalol69.0 µM
Bepridil0.3 µM
Amiodarone27.9 nM
Quinidine0.3 µM
Ziprasidone0.2 µM
Cisapride26.1 nM
Verapamil0.3 µM
Our expertise in a few numbers
64 %
of the last 200 compounds tested in a hERG non-GLP study performed at PhysioStim have shown at least 30% inhibition on hERG channel at their highest concentration
43 %
of the last 100 compounds tested in a hERG GLP study performed at PhysioStim have shown at least 30% inhibition on hERG channel at their highest concentration
Request a quote