Human Cardiomyocytes aconitine resveratrol
Effects of resveratrol on aconitine-induced cardiac arrhythmia in human induced pluripotent stem cell derived cardiomyocytes
R. Printemps, S. Guilbot, M. Le Grand, PhysioStim, Zone Industrielle de Brénas, 81440 Lautrec, France
Resveratrol, a natural ingredient of grape skin, has been demonstrated to exert protective effect on the cardiovascular system. The use of aconitine, the main effective ingredient of a well-known medical herb widely used in traditional Chinese medicine (aconitium), is associated with severe cardiovascular toxicities including tachyarrhythmia and hypotension. The aim of the present work was to evaluate the potential cardioprotective action of resveratrol on aconitine-induced arrhythmia in human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs). To this end, aconitine and resveratrol were tested alone first before evaluation of the co-administration of both compounds to finally assess the impact of a post-treatment of resveratrol after aconitine administration or a pre-treatment of resveratrol before aconitine-induced cardiac arrhythmia.
Methods
Human Induced Pluripotent Stem cells derived cardiomyocytes (iPSC-CMs)
- iCell® Cardiomyocytes2 were supplied by Cellular Dynamics (a Fujifilm company) and seeded on 48 wells plate following manufacturers instruction and analyzed using the xCELLigence RTCA cardio ECR platform (simultaneous measurement of MEA and impedance).
- 4 to 6 days after seeding, iCell2 cardiomyocytes were exposed to different concentrations of aconitine, resveratrol as well as the combination of both compounds, whether by co-administration or by pre-treatment or post-treatment with resveratrol.
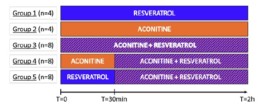
- Data are expressed as Mean ± SD and the first 2h of treatment are presented. This study is composed of 5 groups and the number of replicates in each group is detailed in the experimental design below.
- The following parameters were monitored: Cell Index (CI), Amplitude of contraction, Beat rate (BR) , Beating period (BP) , Individual Beating Duration (IBD), Field Potential Duration (FPD) and FPD corrected by Fridericia (FPDc), Spike amplitude, Beating Rhythm Irregularity (BRI).
Experimental design
Results
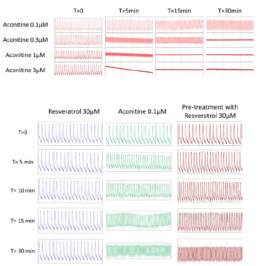
- After 30min, the cells stopped beating with aconitine 0.3µM, 1µM and 3µM.
- Several concentrations of resveratrol were evaluated (0.1µM, 1µM, 10µM and 30µM), no major modification of all the parameters recorded was observed (data not shown).
- Aconitine 0.1µM will be the concentration selected to induce arrhythmia.
- Resveratrol 30µM will be evaluated for its antiarrhythmic capacity to reverse aconitine’s effects.
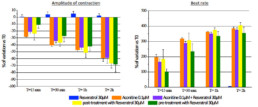
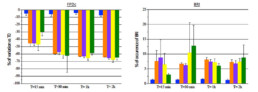
- Tested alone, resveratrol 30µM had no effect on hiPSC-CMs while aconitine 0.1µM time-dependently ↘ amplitude of contraction (-40% after 30min) and FPDc (-60% after 30min) and ↗ Beat rate (+318% after 30min) and BRI (x5 times vs resveratrol).
- Compared to aconitine 0.1µM alone, co-administration of aconitine 0.1µM + resveratrol 30µM had no effect on FPDc and BRI and only slightly lowered effects on amplitude of contraction (∆= -6% after 30min) and Beat rate (∆= -29% after 30min).
- Post-addition of resveratrol 30µM after 30-min exposition to aconitine 0.1µM did not reduce aconitine 0.1µM effects (Amplitude of contraction: ∆= -4%, Beat rate: ∆= -11%, FPDc: ∆= +1% after 30min respectively).
- A 30 min pre-treatment with resveratrol 30µM ↘ aconitine’s effects on all parameters (amplitude of contraction ∆= -17%, beat rate ∆= -93%, FPDc ∆= -15%, BRI ÷3.8 after 15min) and the occurrence of aconitine’s normal effects are delayed by 30min.
conclusion
- Exposition of hiPSC-CMs to aconitine induce a massive increase of beating frequency and a decrease of amplitude of contraction.
- In the same time, resveratrol was not able to suppress aconitine-induced arrhythmia in hiPSC-CMs. Resveratrol was only able to slightly reduce and also delay the occurrence of the maximum effects observed in the presence of aconitine when administrated as a pre-treatment.
- This multi-parametric assay is particularly relevant for the evaluation of drugs’ anti-arrhythmic properties in a model of cardiac arrhythmia in Human iPSC derived cardiomyocytes.
Please fill out the form below to receive the poster
Human Cardiomyocytes Pentamidine Pilsicainide
Evaluation of two hERG channel trafficking modulators, pentamidine and pilsicainide, on stably transfected cell line and human induced pluripotent stem cell-derived cardiomyocytes
R. Printemps1, S. Guilbot1, M. Morton2, M. Le Grand1 – 1 PhysioStim, Zone Industrielle de Brénas, 81440 Lautrec, France | 2ApconiX Ltd, 3F68 AlderleyPark, Cheshire, SK104TG, United Kingdom
hERG trafficking inhibition is known to be one of the mechanisms of indirect hERG inhibition, resulting in QT prolongation and lethal arrhythmia. An increasing number of drugs have been shown to interfere with hERG trafficking. Pilsicainide is a widely used antiarrhythmic agent (class Ic) especially for treating atrial fibrillation. This compound has been evaluated on hERG channel current expressed in stably transfected mammalian cell line as well as on human induced pluripotent stem cell derived cardiomyocytes (iPSC-CM) and compared to pentamidine, a well-known hERG trafficking inhibitor.
Method
Ion channel data
- Electrophysiological recordings of hERG, Nav1.5 (peak), Ito and Iks currents were made from Chinese Hamster Ovary (CHO) cell lines while Cav1.2 currents were made from Human Embryonic Kidney (HEK-293) cell line. All cell lines stably expressed the full-length ion channel. Single cell ionic currents were measured in whole-cell configuration at room temperature (21-23⁰C) using a Patchliner (Nanion Technologies).
- For acute study, cells were clamped at a holding potential of -80mV before a depolarizing step appropriate for each channel. Six Point concentration-response curves were generated by cumulative addition of compound and steady-state inhibition was achieved before the next concentration of compound was added.
- During chronic exposure, hERG cells were exposed during 24h to different concentrations of pentamidine or pilsicainide and then hERG current amplitudes were recorded.
- Data are expressed as Mean ± SEM. For acute study, experiments were replicated 4 times while during chronic exposition, the number of experiments ranged from 28 to 44 for pentamidine, and between 90 and 165 for pilsicainide.
Human Induced Pluripotent Stem cells derived cardiomyocytes (iPSC-CM)
- iCell® Cardiomyocytes2 were supplied by Cellular Dynamics (a Fujifilm company) and seeded on 48 wells plate following manufacturers instruction and analyzed using the xCELLigence RTCA cardio ECR platform (simultaneous measurement of MEA and impedance).
- 4 to 6 days after seeding, iCell2 cardiomyocytes were exposed during 48h to different concentrations of pentamidine and pilsicainide.
- The following parameters were monitored: Cell Index (CI), Amplitude of contraction, Beat rate (BR) , Beating period (BP) , Individual Beating Duration (IBD), Field Potential Duration (FPD) and FPD corrected by Fridericia (FPDc), Spike amplitude, Beating Rhythm Irregularity (BRI).
Results
Ion channel experiments
Ion channel panel, acute exposition:
hERG channel
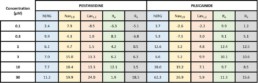
- No acute effect of pentamidine on hERG, Nav1.5, Cav1.2, Iks and Ito.
- Chronic exposition to pentamidine decrease the amplitude of hERG current (estimated IC50 = 9.0µM)
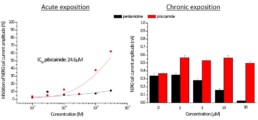
- Acute effect of pilsicainide on hERG channel (estimated IC50 = 24.6µM) but no acute effects on Nav1.5, Cav1.2, Iks and Ito.
- Chronic exposition to pilsicainide increase the amplitude of hERG current (≈+50%) whatever the concentration tested.
Ion channel panel, acute exposition:
iPSCs cardiomyocytes

Pentamidine

Pilsicainide

- No acute effect of pentamidine on each parameter during the first 2 hours. From 6h to 24h, pentamidine 30 μM time-dependently ↓ amplitude of contraction, BR and CIand ↓ FPDc (+123% at 24h) and BRI (at 12h). After 48h, the cells stopped beating with pentamidine 30μM.
- Acute effects of pilsicainide conduct to a concentration-dependent ↓ of BR (-28% at 10µM) and an ↑ of FPDc (+28% at 10µM) with maximum effect after 15 min and sustained through 48h. Pilsicainide from 1 to 10µM had no effect on amplitude of contraction and CI. The cells stopped beating immediately after pilsicainide 20µM addition.
conclusion
- Absence of pentamidine’s acute effects on hERG current in CHO cells is confirmed in Human iPSC-CM. Indeed, no modification of FPDc was observed before 6h of treatment at the highest concentration tested (30 µM).
- On the other hand, the increase of hERG trafficking observed on CHO cells by pilsicainide was not confirmed in iPSC-CM as FPDc was prolonged after short-term exposition and maintained during time.
- This predictive and sensitive assay is particularly relevant for long-term cardiotoxicity assessment in Human iPSC derived cardiomyocytes.
Please fill out the form below to receive the poster